The Implementation of HTM 01-01 at STERIS Instrument Management Services
This article provides an overview of the implementation of the HTM by STERIS Instrument Management Services (STERIS IMS) over the past year.
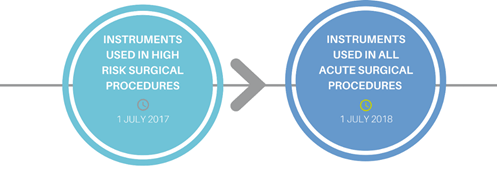
Released in July 2016, the Health Technical Memorandum (HTM) aims to improve patient safety, clinical effectiveness and patient experience by encouraging health care providers and suppliers to adhere to best practice guidelines set by the Department of Health. The HTM became applicable for high risk instruments in July 2017 and in July this year it should be applied to all surgical instruments subject to a decontamination and sterilisation process.
This article provides an overview of the implementation of the HTM by STERIS Instrument Management Services (STERIS IMS) over the past year. We review progress, outline current challenges and discuss the approach moving forward. We have chosen to focus on three of the main recommendations in the new HTM which are applicable to our services; monitoring of protein levels, monitoring the efficacy of the automated wash process and the importance of keeping instruments moist to improve the decontamination process.
As a decontamination and sterilisation service provider, STERIS IMS have utilised this past year to establish the most appropriate methods in terms of selected sampling approach and appropriate trending and analysis of collected data. In June 2017 STERIS IMS implemented the use of protein detection and process challenge devices across all 20 UK facilities.
The HTM 01-01 became applicable for instruments used in high risk procedures in July 2017 and from July 2018, this guidance will impact all facilities reprocessing surgical instruments used in acute procedures.
Despite only being applicable for high risk instruments up until this Summer, STERIS IMS took this approach to ensure consistency across our sites. It also enabled us to compile a large dataset to analyse and allowed time for taking actions to improve the automated wash process if required.
Protein testing
The HTM recommends testing difficult to clean instruments after the automated wash process to establish residual protein levels. At STERIS IMS, this test is carried out in the clean room as part of the release from the washer disinfector.
There are several protein test technologies and methods available on the market. Given certain concerns raised regarding the device and technology suggested in the HTM, STERIS IMS have selected to implement the use of STERIS ResiTest™-Pen to detect protein directly on the surface of individual instruments. These tests can detect protein at levels down to ≤1 µg, which conforms with the suggestions made in HTM 01-01 (≤ 5 µg). The STERIS ResiTest™-Pen was selected after a comprehensive internal study was completed which compared commercially available products based on several criteria. The HTM also suggests that the limit for neurosurgical instruments should be lower than 5 µg, but this remains unspecified.
The HTM 01-01 states that swabbing has been proven to be an ineffective method. The data used to denounce swabbing came from a paper entitled Critical evaluation of ninhydrin for monitoring surgical instrument decontamination (Nayuni et al. 2013). The paper points out correctly that ninhydrin is not sensitive to the 5µg level, however it then goes on to associate swabbing with this lack of sensitivity – the evidence used is that a swab left a remaining 30% of BSA, meaning that 70% was recovered. Subsequent studies have proven that ‘in situ’ techniques have detected less than 50% of a known volume of protein. (Study by Health Facilities Scotland – 2016).
It is evident that both the in situ and swabbing method have both debated in terms of applicability and accuracy. Swabbing is however a well-developed and thoroughly tested method and has several advantages. In comparison to an in situ method it is quick, convenient and requires no re-washing of tested instruments. It can also be used of a wide variety of instruments regarding of size or shape of the instrument surface. A key point to remember is that whether you choose in situ or swabbing as the method for protein testing, there is a need to be able to clearly justify why the selected approach is suitable and effective.
Regardless of which test method or technology is applied, it is also of relevance to ensure all technicians receive training, which enables them to identify the correct instruments as well as use the test without compromising the result. The best way to ensure consistency is to incorporate the testing into the current standard operating procedures and to provide practical training and demonstration to all applicable staff. As for any addition to your current operating procedures, ensure to keep a record of this training on file.
Also keep in mind that if you are utilising dual detergents for the same washer you need to complete the required quantity of tests for each detergent type. This also applies if the washer has different cycle types utilising the same detergent (standard instruments, gentle cycle, anaesthetic/lumen etc)
Recording results and collating data
If your sterile services department (SSD) is managed internally, it might be sufficient to record the protein results manually (but do keep in mind that, for any trending purposes, especially if you are using a fully quantitative protein level measurement technique, you will need to input this to a digital system to enable trending and effective monitoring).
For recording the test data, you should look at recording at a minimum the following:
- The batch and expiry date of any consumables and reagents used (such as the test itself if this is disposable)
- The washer disinfector the instrument was processed in – to be able to determine any correlation between protein levels and individual machines, you may also want to record the cycle number
- The detergent used (if this varies)
- The instrument name and individual instrument identification (or tray ID) – to be able to trend protein levels across certain instrument types or specific instruments
- The date the test was carried out
The requirement to test 50 instruments per quarter, per washer disinfector does not specify intervals or frequency, it is therefore up to each SSD or service provider to determine how to obtain 50 test results per quarter per machine. To gain a representative sampling size it is, for example, not recommended to test 50 instruments during the same day; there may be variations over time which can be a result of external factors such as the loading of the machines. Do keep in mind that if a washer is out of use due to maintenance or breakdown you may need to increase the test frequency to obtain 50 instruments per quarter.
If you are managing a single facility, a simple Excel sheet might be suitable for collating and trending the data. If you are using a protein measurement technique providing a quantitative result, this can be analysed using statistical process control methods – appendix B of HTM 01-01 part A provides a good overview of how to set up such an Excel sheet. For semi quantitative test results, it is appropriate to trend and analyse based on pass/failure rates and to display the data showing several parameters which enables you to uncover any patterns or trends.
STERIS IMS manages a total of 20 facilities across the UK and therefore needs a consistent, centralised approach to collating and analysing data. We collect data using an online database where all test details are inputted electronically. By gathering data from all 20 facilities we can combine a large set of data to uncover trends based on a wide range of parameters.
Process challenge device testing
The HTM has modified the recommendations for monitoring of washer disinfectors and indicates the use of a process challenge devices.
Different to the protein testing, the guidance details required frequency for this test (daily). There are several commercially available process challenge device tests for washer disinfectors, one of them is the Browne STF , which is consists of a single use test strip and a reusable holder which are assembled and placed in the most challenging part of the washer disinfector.
The HTM does not offer detailed guidance regarding how to use the process challenge device and this may differ depending on selected product.
For using the Browne STF, STERIS recommends taking the following approach:
- Initially, it is recommended that the current situation is established by placing PCD’s on each different rack
(5 racks for a 15 DIN washer). The test should be placed such that they cover that back, front and centre of the chamber - Run the washer on a normal cycle but without instruments
- Review the test strips at the end of the full cycle, if any soil remains the washer should be reviewed for issues such as blocked jets, spray arm rotation or other parameters which may have caused the failure
Carrying out this initial test with an empty chamber prior to commencing the daily routine testing will provide a good understanding of the current performance of the machine.
One of the main challenges is to ensure consistency in the test method and in this case, it is mainly in form of the location of the test inside the washer disinfector. As for the protein test, it is of importance to ensure that all relevant information is recorded to enable trending and data analysis. Factors such as Washer type, Washer ID, cycle number and detergent used should be recorded for monitoring and trending purposes. As with the protein testing, remember to carry out daily tests for each detergent or different cycle type, if you have a washer disinfector that utilises dual detergents/cycles.
The results from the PCD testing have been interesting for STERIS IMS given that we have seen quite a wide range of results and variance between different washer disinfector types and detergents used across our facilities. One point of interest to note is in relation to the introduction of this test is that the process challenge device is only introduced as a daily test but does not actually form part of the validation of the machines, where the ‘Edinburgh soil test’ or similar is the recommended process challenge method.
The main challenge in terms of applying the HTM guidelines is to determine actions and evaluate risks based on failures and trends. The HTM does not prescribe an approach for managing failed testing or improving the efficacy of the decontamination process based on recommended protein and PCD testing other than suggesting washer cycle optimisation be carried out. It is therefore up to the SSD to determine actions after analysing collected data.
Managing failures
As an immediate action arising from a failed result, instruments can simply be re-washed through the washer disinfector, or for a failed PCD testing to re-run the cycle. However, if you are experiencing repeated failures this is not a sustainable method and investigating how to optimise the efficiency of the cycle by modifying machine parameters can be a better approach. These types of modifications may include increasing the cycle time, as an example. Any modifications to the cycle parameters will have to be validated by an accredited engineer.
Protein test failures can be the result of several factors such as for example insufficient manual cleaning, detergent, loading of the machine or poor machine performance. Any failures should be managed utilising a risk-based approach in accordance with ISO 14971:2012. It is also useful to identify any potential trends across failures taking factors such as, instrument type, external factors (such as loading of the machine) or detergent into consideration. Remember to document corrective actions taken to ensure compliance to ISO 13485.
It is also of relevance to ensure that the recommendation to keep instruments moist after procedures and during potential transport is implemented. As recommended in the HTM, this should now be a part of the decontamination cycle and should be initiated right after a procedure is completed.
There are several products and methods available to keep instruments moist during transport to the SSD, such as the Medisafe duck bag/kit, the STERIS Pre-klenz™ spray or using swabs and sterile water. A recently published article in the ‘Journal of Hospital Infection’ concluded that, using a pre-cleaning agent improves the cleaning efficacy and should be used to reduce the risk of residual proteins (Smith et al. 2018) As mentioned in the HTM 01-01, prions are especially hydrophobic and tend to adhere to surfaces when dry, it is therefore important to ensure a moist environment is maintained during the time from the operating theatre to the initiation of the decontamination process.
While the requirement to use protein and PCD testing to monitor and trend protein levels is mainly the concern and responsibility of the sterile services departments, the recommendation to keep instruments moist to aid the washing process needs to be initiated in theatres immediately following completion of procedures. This illustrates the need to take a collaborative approach to ensure the effectiveness of the decontamination is maximised and to reduce the risk of contamination by reducing protein levels.
At STERIS Instrument Management Services, we aim to implement best practice guidelines and consistently drive improvements to increase patient safety. We consistently monitor the market for technological advancements to identify innovations which can improve our services
Bibliography:
Critical evaluation of ninhydrin for monitoring surgical instrument decontamination N.K. Nayuni a, E. Cloutman-Green b, M. Hollis b, J. Hartley b, S. Martin c, D. Perrett a; Journal of hospital infection 84 (2013) 97 – 102.
Reducing the risk of iatrogenic CJD by improving the cleaning of neurosurgical instruments. Smith A, Winter S, Lappin D, Sherriff A, McIvor I, Philp P, Suttner N, Holmes S, Stewart A. Journal of Hospital Infection (2018), doi: 10.1016/j.jhin.2018.03.001




